The rapid decline of various bee species—dubbed “the plight of the bumblebees”—has triggered widespread policy discussions about pollinator protections in the United States (“U.S.”). These discussions resulted in fresh developments in a few areas of law aimed at pollinator protection, including the Fish and Wildlife Service (“FWS”) listing the rusty patched bumble bee as an endangered species on March 21, 2017.[2] The rusty patched bumble bee is the first bumble bee protected by the Endangered Species Act (“ESA”). The recent listing is one of the only federal legal protections afforded to any bee species.
The rusty patched bumble bee’s listing introduces new legal questions and presents opportunities to enhance the management and protection of pollinators. The FWS has never managed an insect species that lives in a colony under the ESA. The biological properties of the rusty patched bumble bee greatly differ from species already managed under the ESA which creates novel legal and management issues for the FWS. Properly resolving these issues is critical to rusty patched bumble bee preservation. It also affects the protection of four other bee species scheduled for evaluation for listing by FWS within the next few years. Proper management of the rusty patched bumble bee is important for the protection of the species, future listed bee species, and the ecosystems and organisms that depend on these bees for survival.

Figure 1: A worker rusty patched bumble bee.[3]
This Note explores the unique issues raised by the rusty patched bumble bee’s listing and discusses how those issues could be resolved for optimum protection of the species and pollinators generally. To begin, this Note provides a broad look at the background biological and policy issues surrounding bees. Next, the Note focuses on the rusty patched bumble bee and discusses the species’ decline, its listing as an endangered species, and its management needs. Then, the Note explores those needs in the context of determining critical habitat and setting incidental take allowances for the rusty patched bumble bee. This Note strives to identify key areas of consideration for critical habitat and takings that are unique to the rusty patched bumble bee. Understanding the unique scientific and legal issues surrounding the rusty patched bumble bee will allow for better management, regulation, and litigation.
I. Importance, Decline, and Protection of Bees
Bees, and other pollinators, are critical to the health and reproduction of both agricultural crops and wild plants. The declining population of multiple bee species endangers both agriculture and the environment. A variety of factors contribute to the decline of bee populations, including habitat destruction, loss of genetic diversity, pathogens and parasites, pesticide use, and global climate change.[4] Accordingly, a variety of legal avenues mitigate causes of decline and preserve current populations.
A. Economic and Ecological Importance of Bees
Bees, and other pollinators, support agricultural development and ecosystem management as they play a critical part in reproduction for most plants. Bees pollinate over eighty-five percent of all flowering plant species.[5] Over 180,000 plant species and 1,200 crops benefit from pollination by bees.[6] Pollination by bees is an indispensable service.
Bumble bee species are particularly important for pollination as they are the only type of insect that can “buzz pollinate.”[7] Buzz pollination requires a bumble bee to latch onto the stamen—the pollen-producing reproductive organ of a flower—and vibrate its wing muscles at a specific frequency to release pollen.[8] Nine percent of plant species require buzz pollination and rely on bumble bees for reproduction.[9] Another unique aspect of their critical role as a pollinator is bumble bees’ higher resistance to cold compared to other bees, allowing them to work earlier in the spring and longer throughout each day.[10] Bumble bees provide valuable pollination services to agriculture and the environment.
Agriculture in the United States relies heavily on pollination by bees, as insects pollinate thirty-five percent of agricultural crops.[11] Insect pollination annually contributes to the growth of an estimated $29 billion worth of crops in the United States.[12] In addition to economic production, food security is also very dependent on pollination by wild insects.[13] Many of the crops that primarily provide nutrients and vitamins critical to humans rely on insect pollination.[14] Tomatoes, blueberries, peppers, cranberries, apples, and other common crops all require buzz pollination and rely on bumble bees.[15] Bees, as a whole, play an integral role in agriculture, and their decline would result in significant economic and human health impacts.
Bees are similarly important to wild plants and ecosystem health. Bees coevolved with plants in their native ecosystems which makes them optimal pollinators. When a general pollinator—such as a bumble bee—declines, associated plants also decline.[16] Some plants even reach the point of extinction,[17] making bee population decline a serious risk to ecosystems across the United States.[18]
Pollination services provided by bees are critical to agricultural and wild plants. The observed decline in bee populations and related decline in pollination services are concerning. Loss of these services would have wide scale economic and environmental impacts.
Certain bee species are declining at an alarming rate. Eight of the forty-six U.S. bumble bee species are in decline across various geographic ranges in the United States.[19] Six of these species—the rusty patched bumble bee (Bombus affinis), the Ashton cuckoo bumble bee (Bombus ashtoni), the American bumble bee (Bombus pensylvancus), and three species of yellow-banded bumble bee (Bombus terricola)—have historic ranges in the eastern part of the United States, and two other declining species—the Franklin’s bumble bee (Bombus franklin) and the western bumble bee (Bombus occidentalis)—have historic ranges in the West.[20] Of these species, the rusty patched bumble bee, western bumble bee, American bumble bee, and yellow-banded bumble bee have declined significantly; their populations fell by seventy-two percent to ninety-six percent across their entire native distributions.[21] Similar bumble bee declines in Europe have already resulted in the localized extinction of twelve European bumble bee species.[22]
Environmental stressors, biological vulnerabilities, and human impacts have all contributed to this decline in bumble bee abundance. One major cause is habitat loss. Hive health and queen production are entirely dependent on pollen availability.[23] Bees have long life cycles and only produce a limited number of new queens for the next season at the end of that life cycle.[24] As such, small deficiencies in resource availability have large compounding impacts on a bee species’ abundance the next year.[25] A bumble bee species’ richness, abundance, and genetic diversity all directly rely on access to thriving and healthy habitats.[26] Small isolated habitats cannot sustain bumble bee populations,[27] and road development has increasingly fragmented viable habitat and nutrient sources.[28] The amount of viable habitat that remains unaffected by development is declining. Sixty-two percent of the United States ecoregions where bumble bees live are vulnerable or critically endangered.[29] As habitats continue to degrade and disappear, bee populations that rely on those habitats for nesting and nutrition will decline.
Global climate change is another factor for bee population decline that relates to habitat loss. Associated changes and variability in local temperatures, precipitation, and extreme weather all change habitat and affect bees’ use of that habitat.[30] Earlier snow melt and later frost events change the time of year that different plants bloom. Flowers that bees rely on at specific times in their seasonal life cycle are blooming earlier in the year. This disconnect between the life cycle of bees and the plants they rely on, known as “phenological mismatch,” affects bees’ ability to collect nutrients throughout their entire life cycle.[31] Habitat changes driven by climate change also affect rodent abundance and distribution, consequently decreasing the amount of abandoned rodent dens that bumble bees use for nesting.[32] Climate change creates additional habitat pressures that hurt hive health and productivity.
Bees also face human-caused chemical threats. Agricultural, urban, and home pesticides all have lethal and nonlethal effects on bees.[33] Spring application of pesticides has the largest impact on bees because pesticides affect a new queen’s ability to establish a colony for the season.[34] In particular, the use of a class of insecticide called neonicotinoids is believed to be directly related to bee decline. The insecticide grew popular in the United States in the early 1990s, which correlates temporally to the beginning of major bee population decline.[35] Studies applied neonicotinoids to bumble bees and observed severe harm to colony growth rates and an eighty-five percent reduction in new queen production.[36] Excessive use and misuse of insecticides directly impact bee populations.
Bees also face threats from increased rates of harmful pathogens and parasites. Wild populations are contracting new diseases from escaped commercial bumble bees at an increased rate.[37] The agriculture industry increased its use of commercial bumble bees for greenhouses.[38] These commercial bees escape and spread pathogens and parasites that wild bees lack resistance to.[39] Bee species in decline are particularly susceptible to infection.[40] The increase in disease for wild bees creates additional strain on already struggling bee colonies.
As bee populations decrease in size and are separated from other populations due to habitat loss and fragmentation, inbreeding issues arise. Inbreeding causes a loss of genetic diversity, which in turn reduces a colony’s ability to adapt to its changing environment and increases its susceptibility to pathogens.[41] Genetic issues particularly affect colony species compared to other species. A colony of bees only produces a small percentage of breeding individuals, so the effective population size is significantly smaller than the actual population size.[42] Additionally, inbred populations are more likely to produce infertile male bees, greatly decreasing the colony’s reproductive success.
Environmental stressors caused by habitat loss and global climate change, human introduction of poisons and disease, and the reduced fitness of inbred small bee populations all contribute to the decline of many bumble bee species. These issues interact with one another, compounding and creating a large and complex problem. The variety of causes of bee decline require a variety of solutions. A holistic solution requires multiple policy and legal strategies.
C. Different Avenues for Legal Protection of Bees
Different regulatory frameworks offer solutions to some of the threats to bee populations. Some of the broader issues, such as habitat loss and global climate change, require regulation of public lands, agricultural development, urban development, carbon emissions, and ecosystem management. It is unlikely these areas would be addressed specifically for bee protection; however, bees would benefit from future policies that mitigate these broad environmental stressors. Other legal avenues have more direct opportunities to immediately strengthen bee protection.
The Federal Insecticide, Fungicide, and Rodenticide Act is the clearest avenue to address insecticide issues.[43] This federal law creates rules regulating and banning the use of specific chemicals as insecticide.[44] The Environmental Protection Agency is currently reviewing neonicotinoids under the Act.[45] Restriction of neonicotinoids would relieve a large threat to the continued decline and loss of bee species.[46]
Pesticide and pathogen issues are also immediately addressable through agricultural management of commercial bees.[47] Both federal and state level agricultural regulation can address the problem.[48] Stricter regulation, management, and inspection of commercial bumble bee operations could both reduce the amount of disease in commercial bee populations and decrease the amount of accidental release of diseased commercial bees into wild populations.[49] Additionally, regulations prohibiting the transportation of diseased commercial bumble bees to areas currently unaffected by disease would greatly decrease the risk of pathogen and parasite exposure for wild bees.[50]
The ESA is another major avenue to provide protection to any listed bee species and their habitat. The purpose of the ESA is to “protect and recover imperiled species and the ecosystems upon which they depend.”[51] The ultimate goal of the law is for a listed species to recover to the point that it no longer needs protection.[52] The ESA defines an endangered species as “any species which is in danger of extinction throughout all or a significant portion of its range.”[53] The ESA mandates that the FWS base a decision to list a species on five factors: “[1] the present or threatened destruction, modification, or curtailment of its habitat or range; [2] overutilization for commercial, recreational, scientific, or educational purposes; [3] disease or predation; [4] the inadequacy of existing regulatory mechanisms; or [5] other natural or manmade factors affecting its continued existence.”[54] Any factor or combination of factors can justify a listing decision.[55] Critical habitat for an endangered species must be determined concurrently with a listing decision.[56] In practice, however, the FWS has the ability to delay or not designate critical habitat for a variety of reasons.[57] The FWS has not yet designated critical habitat for any of the listed bee species.
The FWS listed seven species of Hawaiian yellow-faced bees in 2016.[58] These solitary bee species were the first and only bee species protected under the ESA until the FWS listed the rusty patched bumble bee in 2017.[59] The FWS intends to evaluate four bee species for future protection under the ESA: (1) the Franklin’s bumble bee and (2) the yellow-banded bumble bee in 2018, (3) the western bumble bee in 2023, and (4) the blue calamintha bee at an unspecified date.[60] Notably, the FWS recently proposed the Franklin’s bumble bee for listing as endangered or threatened in August 2019 and a final decision is pending.[61] Listed species receive federal protection under a number of different sections of the ESA.
Once listed, a bee species receives several general federal protections. Section 7(a)(2) of the ESA requires a federal agency to consult with the FWS to ensure any agency action “is not likely to jeopardize the continued existence of any endangered species . . . or result in the destruction or adverse modification of habitat of such species which is determined . . . to be critical.”[62] The consultation process triggers various administrative processes designed to protect endangered species for any major federal agency action.
Section 9 expands endangered species protection to the private sector and makes it illegal for any person—public or private—to “take” any endangered species.[63] The ESA defines “take” as “to harass, harm, pursue, hunt, shoot, wound, kill, trap, capture, or collect, or to attempt to engage in any such conduct.”[64] The outward boundaries of “take” have been widely litigated,[65] particularly within the context of adverse modification of habitat. There are limits and exceptions to the take prohibition and its protection. Section 7 allows for the incidental take of a listed species by a federal action up to a specified “take limit.”[66] Section 10 of the ESA allows the FWS to issue permits excepting a private action from the take prohibition.[67] The ESA requires a habitat conservation plan for a Section 10 take permit.[68]
The ESA has a citizen suit provision which allows any citizen to sue any person or agency for violations.[69] This allows parties dedicated to the protection of bees to take direct legal action in private and public development that impacts listed bee species.
Additionally, it allows groups to actively protect rusty patched bumble bee habitat as a way to provide indirect protection to unlisted bee and plant species that share that habitat.[70] The ESA could be a useful means to make immediate change and conservation efforts for the most threatened bee species, listed or otherwise.
Bees are recent additions to the ESA and the FWS has never managed a colony insect species under the ESA. Historically, ESA protections offered for insects and other invertebrates—animals lacking a backbone, including insects, snails, clams, arachnids, crustaceans, and coral—have been lackluster.[71] Although the drafters of the ESA intended to protect more than just famous megafauna,[72] the ESA seems to protect vertebrate species more than invertebrate species.[73] Bias in favor of vertebrate species is observable throughout the entirety of the ESA’s implementation, starting with listing. Insects comprise seventy-two percent of global animal diversity but represent less than five percent of the endangered species list.[74] A large contributing factor to the low number of insects is a general lack of knowledge about invertebrates. Less than three percent of invertebrate species have enough data to be evaluated for listing due to a widespread lack of knowledge and specific research.[75] There is also a clear disparity in the effectiveness of management of vertebrate and invertebrate species under the ESA. As of 2001, twenty-nine vertebrate species recovered from endangered status compared to two recovered invertebrate species, none of which were insects.[76] While the ESA certainly provides some protection to the listed bee species, it does not line up perfectly with insect management needs.
II. The Rusty Patched Bumble Bee and the Endangered Species Act
The listing of the rusty patched bumble bee is of particular legal interest and significance for both general pollinator protection and the ESA. The listing has broader implications for other pollinators as it is one of the only federal legal protections specific to bees. Successful management of the rusty patched bumble bee under the ESA would provide important expectations and norms for managing the four bee species that the FWS will evaluate in coming years. The listing also highlights specific, previously unanswered questions about how provisions relating to critical habitat and the take work for a eusocial—or colony—species.
A. Rusty Patched Bumble Bee Decline
Historically, the rusty patched bumble bee was an abundant and common bumble bee species in the eastern United States.[77] The species is a general pollinator known and named for a small orange patch on the back of worker bees. The rusty patched bumble bee’s historic range extended from the Appalachian Mountains in the East to the Midwestern prairies to the West, and from the lower parts of Canada down to the state of Georgia.[78]
This once-expansive range is significantly smaller today. The species has disappeared from seventy-five percent of its historic range.[79] Where the rusty patched bumble bee previously flourished in thirty-one states and provinces in the United States and Canada, it now only occupies fourteen.[80] Tallgrass prairies—the rusty patched bumble bee’s preferred ecosystem—have declined by more than ninety-eight percent during the last few decades.[81]
Figure 2: “Rusty patched bumble bee range map showing the current distribution. Dots represent counties with a rusty patched bumble bee occurrence since 2000. The Xs represent counties with the historic occurrences only.”[82] 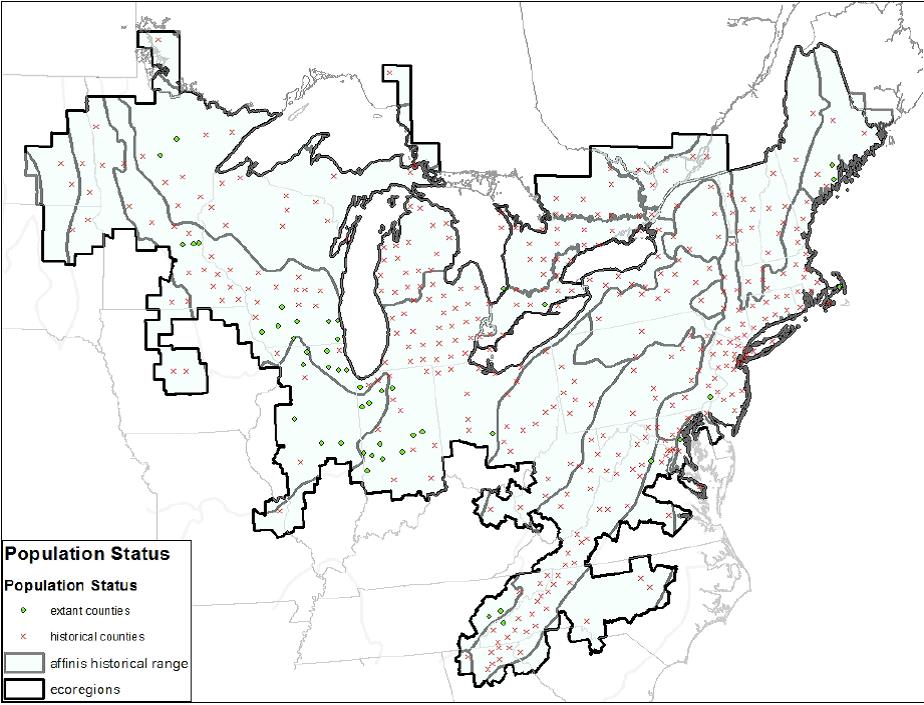
The rusty patched bumble bee’s population has decreased to an even more alarming degree than its range. The species’ relative abundance has decreased by ninety-five percent from historic numbers.[83] Healthy populations of bumble bees contain tens to hundreds of colonies, each of which has several hundred individuals.[84] However, in ninety-five percent of the known populations, scientists documented five or fewer individuals; the maximum number of individuals found at any site was thirty.[85] The recent colony data comes from surveys conducted in the early 2000s and many of those colonies have not been reconfirmed. As much as forty to seventy-five percent of those colonies may have been lost before the rusty patched bumble bee gained endangered status.[86]
The once abundant rusty patched bumble bee has been completely lost from large swaths of its historic range. The few populations that still exist are dangerously small and at risk of further decline.
B. Listing as an Endangered Species
The FWS listed the rusty patched bumble bee as an endangered species on January 11, 2017.[87] After a brief delay, the species gained official protection under the ESA on March 21, 2017.[88] The FWS based its listing decision on the rusty patched bumble bee’s inability to cope with physical and biological environmental changes, small population size, and the degradation and loss of many of the ecoregions the species relies on.[89]
Critical habitat was not designated at the time of listing.[90] The FWS stated that it possessed insufficient information to perform the required analysis for critical habitat designation.[91] The listing referenced ongoing biological information assessments that would supplement current data,[92] but the FWS did not provide a timeline for producing a critical habitat determination based on the supplemented information.[93] Critical habitat could still be designated, which would have specific impacts for Section 7 agency action protections. However, it is common for species to never receive critical habitat designations.[94]
The listing illustrated the types of actions that could constitute a take. The listing stated that a take may be:
(1) Unauthorized handling or collecting of the species; (2) The unauthorized release of biological control agents that attack any life stage of the rusty patched bumble bee, including the unauthorized use of herbicides, pesticides, or other chemicals in habitats in which the rusty patched bumble bee is known to occur; (3) Unauthorized release of nonnative species or native species that carry pathogens, diseases, or fungi that are known or suspected to adversely affect rusty patched bumble bee where the species is known to occur; (4) Unauthorized modification, removal, or destruction of the habitat (including vegetation and soils) in which the rusty patched bumble bee is known to occur; and (5) Unauthorized discharge of chemicals or fill material into any wetlands in which the rusty patched bumble bee is known to occur.[95]
Although the list does not mandate what a take is, it still demonstrates that the FWS intends the ESA to protect the rusty patched bumble bee from direct human impacts—improper pesticide use and disease spread by commercial bees—as well as environmental impacts and habitat degradation.
Although listing triggers some legal protections—such as establishing critical habitat and limiting the take of a species—under the ESA, the actual amount of protection provided to a species depends on how the FWS specifically implements those protections. All species have unique needs that require attention for successful management. Meeting those needs is a process that extends long after the initial listing of a species.
C. Unique Management Needs of the Rusty Patched Bumble Bee
The rusty patched bumble bee possesses a number of biological qualities that impact its management under the ESA. While some are shared by other species already managed under the ESA, others are unique to eusocial insects—including some types of bees, hornets, and ants—and have never been addressed under the ESA. The FWS will need to understand and address these qualities in order for the rusty patched bumble bee to recover.[96]
1. The Rusty Patched Bumble Bee’s Life Cycle
The annual life cycle of bumble bees is critical to understanding the species’ different needs at different stages in the cycle and at different times throughout the year. In early spring, a queen emerges from hibernation.[97] The queen spends the spring and early parts of the summer collecting pollen and then establishes a colony in the late summer.[98] The species is particularly vulnerable before establishing a colony.[99] At this stage, each individual queen represents an entire colony, and the loss of a queen bee means the loss of an entire colony. The ability to form a colony also depends on a queen’s ability to gather enough nutrients. Changes in resource availability in the spring and early summer have compounding impacts on later colony health and productivity.
Once a queen establishes a colony, she begins to produce daughter workers. Those drone bees go out and forage for pollen for the rest of the season.[100] Healthy rusty patched bumble bee colonies have relatively large colony outputs, with an observed average of over 1,000 workers in one season.[101] Once the foundress—the establishing queen—has produced enough drones to fill immediate needs and sustain the growing colony, the queen will begin to produce male bees and new queen bees intended for breeding. A healthy colony produces close to 200 new queens over the life of the colony.[102]
In the fall, the new queen and male bees leave the colony to breed with new queens and males from other colonies.[103] Once a new queen has successfully mated, she goes into hibernation for the winter.[104] The rest of the colony—the foundress, drones, and male bees—dies.[105] Come spring, the new queens emerge from hibernation and establish their own colonies using the genetic information gathered the previous fall.
A bumble bee species possesses several unique biological qualities inherent to this life cycle and its eusociality. One such quality is the internal genetic division of the species into different roles. Queen bees are responsible for founding colonies, and as such have specific abilities to breed, lay eggs, hibernate, and operate individually while establishing a colony.[106] Worker bees, on the other hand, are responsible for food collection, colony defense, and feeding the colony’s young.[107] Male bees’ sole function is to mate with new queens and leave the nest upon reaching maturity.[108] The different functions of the bees create a difference in relative importance to each type of bee at different times. Killing a queen bee is inherently different than killing one worker bee. Harming a worker bee in the summer when a colony is young and needs support is different than harming a worker bee in the late fall when the colony is old and has already produced its reproductive individuals.
Habitat needs also directly link to the specific life cycle of the rusty patched bumble bee. The rusty patched bumble bee’s historic range is associated with a broad range of habitats including prairies, agricultural lands, marshes, forests,[109] and sometimes residential parks and gardens.[110] Although the rusty patched bumble bee has historically lived in a variety of habitat types, an area must meet specific needs in order to be a suitable habitat. The rusty patched bumble bee has a narrow climatic niche associated with the northern parts of the United States. In the southern parts of the species’ historic range, colonies only occur at high elevations.[111] This narrow climatic niche makes the rusty patched bumble bee vulnerable to climatic changes since shifts in average temperature of the species historic range would make it unviable. In addition to fitting in a specific climatic niche, suitable habitat must also meet foraging, nesting, and hibernating needs.[112] Habitat alteration that interferes with any one of these requirements threatens the rusty patched bumble bee. Residential, commercial, and agricultural development all degrade, replace, or break-up suitable habitat.[113]
Foraging and nutrient availability causally relate to colony health and are critical elements of habitat needs. Floral abundance, diversity, and phonological timing are all essential elements of the rusty patched bumble bee’s foraging needs. Bumble bees need access to a lot of nutrients to sustain a colony; scientists linked floral abundance in prairies directly to bumble bee abundance and species diversity.[114] The rusty patched bumble bee needs suitable foraging habitat for an estimated 1,000 meter or higher radius surrounding a colony.[115] That requirement could be higher as better studied bumble bee species closely related to the rusty patched bumble bee travel up to 2,500 meters away from a colony to forage.[116] The rusty patched bumble bee is a general forager and relies on a wide range of plants for pollen. Scientists documented the species visiting at least sixty-five different genera of plant.[117] The species is short-tongued and therefore benefits from high numbers of open flowers with short corollas—the part of a flower’s petal that surrounds and protects the plant’s reproductive organs.[118]
In addition to needing an abundance and variety of flowers, bumble bees also need access to blooming flowers at all times between April and October.[119] Floral availability is particularly important when new queens are attempting to establish a colony in early spring while few flowers are in bloom.[120] Seasonal and climatic variations can change the timing of blooming flowers and create stressors for the rusty patched bumble bee.[121] The rusty patched bumble bee’s specific foraging needs are critical to its preservation.
Nesting and hibernation sites are also important elements of the rusty patched bumble bee’s foraging needs. The rusty patched bumble bee usually nests underground, often in abandoned rodent nests or other existing cavities.[122] The species occasionally nests above ground in a tree stump, dead wood, or clumps of grass when suitable nesting sites are unavailable.[123] Environmental changes that affect the abundance of rodents or the availability of grassland impact the rusty patched bumble bee’s ability to find nesting sites.[124] A foundress can travel up to five kilometers to find a suitable nesting site;[125] preserving nesting habitat therefore requires large areas of suitable habitat. Additionally, the nests are vulnerable to any industrial or agricultural development.[126] Large machinery could destroy or indirectly impact a colony’s nesting site by changing the immediate surrounding area.[127] Queen bees hibernate by burrowing down into undisturbed soil, which is similarly vulnerable to industrial or agricultural development during the winter.[128] The nesting and hibernation requirements add to the complexity and specificity of preserving suitable habitat for the rusty patched bumble bee.
The specific breeding strategy implemented by eusocial insects also produces unique management considerations. The small size of the remaining rusty patched bumble bee population makes genetic diversity, inbreeding, and specific issues with sex determination significant threats to the species preservation and recovery.[129] The majority of a bee colony are non-breeding worker bees, so the effective population size—number of breeding individuals—is much smaller than the actual population size, which increases the rate of inbreeding and associated genetic defects.[130] The foundress is the only reproducing female and usually only mates once, so an entire colony stems from only one breeding pair.[131] As such, inbreeding effects compound quickly when bees breed within their own colony or closely related colonies. Bumble bee populations that have less than fifteen square kilometers of habitat are more likely to show signs of inbreeding.[132] Loss of genetic diversity reduces a population’s ability to adapt to environmental changes and increases susceptibility to pathogens and parasites.[133]
Inbreeding also relates to an issue called the “diploid male extinction vortex,” where a colony produces infertile or nonviable male bees.[134] A foundress produces a female bee by laying a fertilized egg and a male bee by laying an unfertilized egg. Female bees are diploids and get two sets of chromosomes—one from the queen and one from the male—while males are haploids and only get one chromosomal set. Inbred bee populations can produce diploid male bees that receive two copies of the queen’s chromosomes.[135] Diploid males are often sterile, but when they do mate with a new queen, she produces sterile triploid offspring.[136] The production of diploid males wastes colony resources and consequently slows a colony’s growth rate, lowers survival, and reduces the number of viable offspring produced.[137] This issue makes bees particularly vulnerable to extinction compared to other species covered by the ESA.[138] The life strategy of the rusty patched bumble bee and other eusocial insects creates unique management needs not yet addressed by the ESA.
III. Maximizing Protection of the Rusty Patched Bumble Bee Under the Endangered Species Act
Designating critical habitat and setting incidental take allowances are two important elements of the ESA that relate to the unique biological properties of the rusty patched bumble bee. Both elements of the law are complicated and situational. Management under the ESA varies among species and populations of species. As such, specific numeric suggestions for the rusty patched bumble bee require immense amounts of detailed technical information and are outside the scope of this Note. Instead, this Note focuses on how the eusocial properties of the rusty patched bumble bee relate to critical habitat designation and setting take limits generally. This Note then identifies areas of opportunity to maximize protections for the rusty patched bumble bee and other eusocial insects.
A. Maximizing Protections for the Rusty Patched Bumble Bee Using Critical Habitat
1. Critical Habitat Determination
The ESA defines critical habitat as land within a species’ current range that possesses the “physical or biological features essential to the conservation of the species and which may require special management considerations or protection.”[139] The FWS defines the species’ current range as “areas used throughout all or part of the species’ life cycle, even if not used on a regular basis.”[140] The ESA also allows the FWS to include areas outside of the current range if “such areas are essential for the conservation of the species.”[141] Specifically, these additional areas are intended to include a species’ former range.[142]
Under the ESA, conservation is synonymous with recovery, so critical habitat encompasses the area needed for a species to recover from endangered status.[143] The Secretary must designate critical habitat to the “maximum extent prudent and determinable”[144] and base that decision, in part, on the “best scientific data available.”[145] However, the FWS must also consider economic impacts and any other relevant impacts of a critical habitat designation.[146] If the impacts outweigh benefits of designating critical habitat, the FWS can choose to not designate part or all of a species’ range as critical habitat.[147]
Critical habitat is important to habitat conservation which is critical to species’ survival.[148] Habitat reduction and degradation are strongly tied to decreases in population size,[149] contributing to several issues for the rusty patched bumble bee that increase its risk of extinction. Habitat loss is one of the most important factors for species extinction.[150] Thirty-six percent of extinctions are caused by habitat loss.[151] Consequently, habitat conservation improves species conservation.[152] Broad ecosystem conservation is particularly important when conservationists are still studying a species and are unsure about its biological needs because it provides a blanket protection for the species without needing a specific understanding of every factor relevant to the species’ conservation.[153] Protections for species under study are relevant to the rusty patched bumble bee, as the FWS chose not to designate critical habitat due to a lack of information.[154] Designation also focuses external conservation efforts on a species habitat, encourages research and understanding of the area, and prevents inadvertent harm to the species.[155]
Critical habitat is also important in the Section 7 consultation process. All major action by federal agencies must avoid the destruction or adverse modification of designated critical habitat.[156] Before taking a major action, an agency is required to consult with a managing agency—often the FWS—to determine whether the action is “not likely to jeopardize the continued existence of any endangered species . . . or result in the destruction or adverse modification of [critical habitat].”[157] If the consultation concludes there is jeopardy to a species or adverse modification of critical habitat, then the Secretary provides “reasonable and prudent alternatives” that prevent violation of the ESA.[158] Critical habitat is one of the only triggers for the consultation process and is the only protection available for unoccupied, but still important, habitat.[159] The absence of designated critical habitat significantly reduces Section 7 protections under the ESA.
Critical habitat designation also leads to legal protection beyond simply triggering the consultation process. A species is more likely to have a recovery plan if it has designated habitat.[160] There is also better task implementation for recovery plans for species with designated critical habitat.[161] Critical habitat designation also increases judicial review of actions affecting an endangered species.[162] Without critical habitat, judicial review for a Section 7 violation only occurs for direct jeopardy to a species.[163] With critical habitat, judicial review for a Section 7 violation is also triggered by harm to that critical habitat.[164] Designated critical habitat also expands protection within the consultation process. Biological opinions that infringe on designated habitat are more likely to find that an agency action causes jeopardy to a species.[165]
Critical habitat designation is effective at protecting endangered species.[166] Species with designated critical habitat are more than twice as likely to begin recovering and less than half as likely to decline further.[167] The biological benefits of preserving habitat, and the added legal protections, make a significant difference in the recovery of an endangered species.
The FWS often hesitates to designate critical habitat for endangered species despite the conservation benefits. The FWS has not designated critical habitat for almost eighty percent of all listed species.[168] Designations are particularly rare for insect species; only forty-two insect species have critical habitat designations.[169] Notably, in the recent proposed listing for the Franklin’s bumble bee, the FWS declined to designate critical habitat for the species as it “would not be beneficial.”[170] The FWS informally practices a policy of disinterest in designating critical habitat, known as the “functional equivalence” policy.[171] This policy flows from the idea that the Section 7 jeopardy protection of a species and the adverse modification protection of critical habitat serve the same function and therefore make critical habitat designations redundant and unnecessary for species protection.[172] Despite the FWS’s disinterest in designating critical habitat, a determination one way or another is still statutorily required within a year of listing a species.[173] A one-year extension is provided when critical habitat is “not determinable,” as is the case for the rusty patched bumble bee.[174] The extension leads to delay in protections that is harmful to a species.[175]
2. Considerations for Designating Critical Habitat for the Rusty Patched Bumble Bee
At the time of listing, the rusty patched bumble bee’s critical habitat was not determinable.[176] The FWS stated “a careful assessment of the biological information is still ongoing, and we are still in the process of acquiring the information needed to perform that assessment.”[177] The critical habitat designation for the rusty patched bumble bee has yet to be added to the FWS’s work plan.[178] The FWS allotted the statutory grace period to make a determination about critical habitat for the rusty patched bumble bee, and consequently the Natural Resource Defense Council filed suit against the agency on January 15, 2019.[179] This designation decision will be critical to the protection of the rusty patched bumble bee. There are opportunities to use critical habitat designation to address many of the species’ unique management needs.
The most important consideration for critical habitat is that it must be designated for the rusty patched bumble bee. The FWS did not assess critical habitat at the time of listing and might not designate critical habitat at all due to the complexity of the issue. There are potentially large economic impacts to designating habitat for a delicate species with a large range. However, economic impacts must be weighed against the conservation benefits. The rusty patched bumble bee’s survival directly depends on habitat. Foraging availability for the species’ entire life cycle, nesting and hibernating site abundance, minimizing inbreeding depressions, and promoting healthy colony growth and reproduction all relate to preserving the remaining habitat that is suitable for the species.[180] The FWS has not been generous with critical habitat designation for insect species.[181] The rusty patched bumble bee needs this additional biological and legal protection for recovery. The FWS also needs to muster administrative speed for making the designation as suitable habitat further degrades while waiting for designation.
Full protection of the rusty patched bumble bee would require an unprecedented critical habitat designation for an insect species. Of the forty-two endangered or threatened insect species with proposed or final critical habitat, many have small and isolated designations based around a few populations.[182] The average critical habitat designation for insects is 6,132.8 acres.[183] Those designations range from 0.3 acres for the Ash Meadows naucorid to 61,973.8 acres for the Quino checkerspot butterfly.[184] Most of the designations are composed of a few swatches in a concentrated area based on a specific population.[185] A comparable critical habitat designation for the rusty patched bumble bee would fail to provide the required level of protection for the species. The current range of the rusty patched bumble bee covers more than a dozen states and cannot be covered by a few small and isolated designations.[186] Minimal protection of existing populations would already create an unprecedented critical habitat designation for an insect. Maximum protection would involve an even larger critical habitat designation of land outside the current range that is “essential for the conservation of the species.”[187] The rusty patched bumble bee’s unique biological needs require an expansive critical habitat designation.
Critical habitat designation should focus on the rusty patched bumble bee’s foraging needs. The purpose of designating critical habitat is to protect areas that contain essential physical and biological features.[188] Floral abundance and variety are a critical biological feature of an area that directly relates to colony health and productivity.[189] Designation should ensure enough flowering species are protected to preserve access to pollen for the rusty patched bumble bee’s life cycle. Special attention should be paid to including floral species that bloom during the spring while a foundress is preparing to establish a colony as a population is particularly vulnerable to long term effects of malnutrition.[190] Each colony is estimated to need one square kilometer up to two-and-a-half square kilometers—roughly 250 to 625 acres for comparison to other insect critical habitat designations—of foraging area.[191] Although the specific numeric size of critical habitat designation will be incredibly site-specific, these facts give an idea of how much space the rusty patched bumble bee needs to meet its floral abundance and diversity needs for foraging. Any designated critical habitat needs to explicitly protect foraging habitat in order to fully protect the rusty patched bumble bee.
The FWS also needs to avoid habitat fragmentation. Habitat fragmented by development reduces the rusty patched bumble bee’s foraging range and the connectedness of a population.[192] The rusty patched bumble bee is particularly vulnerable to negative genetic effects caused by small populations and inbreeding.[193] A large number of colonies need to be connected to preserve genetic diversity and maintain a healthy population.[194] The geographic range required for a genetically healthy colony is fifteen square miles—9,600 acres—which is significantly greater than the range needed for foraging.[195] The small isolated patches of critical habitat designated for other insects would fail to protect the rusty patched bumble bee from further habitat fragmentation and smaller population sizes. Instead, an effective critical habitat designation would need to provide larger swaths of land and seek to connect geographically close colonies in order to preserve an entire population rather than a few separate and inbred colonies.
Preserving nesting and hibernation habitat is also necessary for conserving the rusty patched bumble bee.[196] The species has specific hibernation and nesting needs for a foundress to survive to the point of establishing a colony.[197] There are no specific ways to pinpoint the abandoned rodent nests and undisturbed soil required by the rusty patched bumble bee. The only way to protect nesting and hibernation habitat is to preserve larger areas of land that are likely to meet these needs. Although the geographic areas designated to protect foraging and genetic diversity might include the physical characteristics needed for hibernation and nesting, there is no guarantee. Nesting and hibernation habitat should be separately and explicitly considered and included in critical habitat designation.
The critical habitat designation should leverage the diversity of viable ecoregion types available to the rusty patched bumble bee to help preserve the large areas needed for foraging and genetic diversity. Grasslands, forests, and marshes have all proven to be suitable habitats for the rusty patched bumble bee under the right conditions.[198] Each provides slightly different foraging, hibernation, and nesting needs and is important to the rusty patched bumble bee, even if grasslands are the preferred ecoregion. Intentionally including forests and marshlands would allow for broader geographic designations needed for foraging and genetic diversity. Considering the rapid decline in grassland ecoregions,[199] inclusion of other viable ecoregions is likely necessary for even minimal critical habitat designations. Failing to include viable ecosystems other than grasslands would inhibit the conservation mission.
A broad critical habitat designation is also necessary to account for uncertainty and variability. “Current” colony sites identified in the listing come from data gathered in the early 2000s.[200] Nest locations change annually and can be far away from the former seasons’ nesting sites.[201] As such, the FWS should not designate small areas centered on the current data for the rusty patched bumble bee colony location. Centering small areas on newly collected data would be equally ineffective as it would only guarantee to protect colonies for one season. The only way to ensure that the area immediately surrounding a colony is protected is to embrace the inherent uncertainty and designate large areas of suitable nesting habitat.
The functional means to address a lot of the unique habitat needs for the rusty patched bumble bee is to designate critical habitat and include as much suitable habitat as possible. Although this recommendation is simple on its surface, it addresses specific management needs for the rusty patched bumble bee. The reasoning behind the recommendation is more nuanced than a blanket call for designating more land as critical habitat for the sake of preserving more habitat. The rusty patched bumble bee needs a lot of land for survival. Large geographic areas are necessary to preserve foraging availability and variety, maximize nesting and hibernation site abundance, avoid fragmentation to preserve genetic diversity, protect multiple viable ecoregions, and account for variability and uncertainty in the species’ site-specific habitat needs. Each of these considerations are necessary for rusty patched bumble bee conservation. Understanding and addressing each of these needs is important in justifying a larger and more expansive critical habitat designation than that designated for other insects.
The critical habitat designation for the rusty patched bumble bee presents an opportunity to expand the species’ protection under the ESA. It also has larger implications for pollinator and environmental protection. Three of the four bee species under consideration by the FWS are bumble bees with similar habitat needs.[202] Proper protection for the rusty patched bumble bee could be precedential for future protected bees. The rusty patched bumble bee is also an umbrella species—a species whose protection contributes to the protection of other species—and protecting its habitat would protect associated floral species, other bumble bee species, and the general ecosystem in the area.[203] A strong critical habitat designation for the rusty patched bumble bee would be a significant positive step in environmental law.
B. Maximizing Protections for the Rusty Patched Bumble Bee Using Take Allowances
The ESA prohibits any individual from taking a listed endangered species.[204] The take prohibition is a powerful protection for endangered species and covers a wide range of activities and situations.[205] The statutory definition of “take” encompasses harassing, harming, pursuing, hunting, shooting, wounding, killing, trapping, capturing, or collecting a protected species.[206] “Harass” includes acts that intentionally or negligently “creat[e] the likelihood of injury to wildlife by annoying it so much an extent as to significantly disrupt normal behavioral patterns which include, but are not limited to, breeding, feeding, or sheltering.”[207] “Harm” is defined by regulation as an “act that kills or injures wildlife,” and includes “significant habitat modification or degradation where it actually kills or injures wildlife by significantly impairing essential behavioral patterns including breeding, feeding, or sheltering.”[208]
Judicial treatment of the take provision established that a take does not have to be intentional and does not have to be a direct application of force.[209] However, an action does need to be foreseeable and a proximate cause to actual injury or death to be a take.[210] When a party violates the take prohibition, that party is liable for up to $25,000 of civil penalties per violation, and in extreme cases, may be subject to criminal penalties under Section 11 of the ESA.[211] The ESA also allows for injunctive relief against alleged future takes.[212] The take provision is set up as a strong statutory protection for listed species designed to prevent and discourage further degradation of endangered species.
However, there are exceptions to the take prohibition.[213] The FWS can set incidental take allowances both for public and private actions. The take must be incidental to some other lawful activity.[214] The take allowance must also be small enough to not jeopardize the persistence of a listed species for public actions.[215] For private actions, it cannot reduce the species’ likelihood of survival.[216]
Section 7 of the ESA builds a take exception into the consultation process for major agency actions. The FWS issues biological opinions at the end of the formal consultation process. These biological opinions include an incidental take limit for the evaluated project that sets the level of anticipated and permissible take.[217] This exception under Section 7 is the most common form used by the FWS to allow incidental takes.[218] Almost no projects are actually stopped through the consultation process.[219] Only around ten percent of decisions go through the formal consultation process.[220] Of that ten percent, over ninety percent find “no jeopardy” and of the other ten percent, ninety percent are resolved through implementing reasonable and prudent alternatives.[221] As such, around ninety-nine percent of the projects evaluated under the formal consultation process produce biological opinions that contain incidental take allowances and permit a specific level of take of endangered species.
Section 10 of the ESA grants the FWS discretionary authority to issue incidental take permits to private parties.[222] The permits require the private party to include a habitat conservation plan designed to mitigate the impacts of the applicant’s project on a listed species.[223] The habitat conservation plan lays out strategies to minimize and mitigate impacts to the “maximum extent practicable.”[224] The FWS evaluates the proposed permit and ultimately decides whether the level of take is acceptable without “appreciably reduc[ing] the likelihood of the survival and recovery of the species in the wild.”[225] There are no objective calculations or guidelines about the allowable quality and quantity of take or amount of mitigation; both features are simply encapsulated by the FWS’s discretion to issue the permit.[226]
The discretionary nature of setting specific incidental take limits makes the practice highly litigated. Multiple cases about the consultation process and incidental take allowances for the rusty patched bumble bee have already been litigated. Most of the cases were dismissed on procedural grounds and never addressed the substantive question of what an allowable take for the rusty patched bumble bee is.[227] However, in Sierra Club v. U.S. Department of Interior, the Fourth Circuit held that the take limits set by the FWS were not enforceable and were therefore arbitrary and capricious in violation of the Administrative Procedure Act.[228] The case involved a biological opinion issued for a pipeline that impacted the rusty patched bumble bee and five other endangered species.[229] The FWS set the take limit as killing one colony and harassing a “small percent” of queen bees from one colony for the entire affected areas for the rusty patched bumble bee.[230] The court found that the limit was not an enforceable standard because a “small percentage” was non-quantifiable, especially when considering the lack of species data for the rusty patched bumble bee.[231] While this case sheds some light on what does and does not constitute a numeric take limit, it fails to answer what an acceptable take limit for the rusty patched bumble bee is. As of now, this question is left up to the careful discretion of the FWS and future litigation.
2. Considerations for Setting Take Allowances for the Rusty Patched Bumble Bee
The rusty patched bumble bee’s specific and unique properties are relevant to setting take limits. Understanding the relationship between the species’ biological properties and the amount of take that is allowable is important as the FWS issues more take allowances and specific numeric limits continue to be litigated.
The remaining rusty patched bumble bee populations are small, unhealthy, and fragile,[232] and cannot afford liberal take allowances. A colony relies on each of its members.[233] Current colony sizes are well below the level of healthy colonies, increasing the individual importance of each bee.[234] While an individual worker bee may not have much importance to a healthy hive with over 1,000 individuals, in a hive that has less than 100 individuals, each worker bee provides for a large percentage of the colony’s foraging and nutrition needs. In small colonies, the individual importance of male and new queen bees also increases substantially. Genetic diversity loss from inbreeding is a particularly potent problem for the eusocial breeding strategy, and the loss of any individual breeding bee compounds the problem.[235] Rusty patched bumble bee conservation depends on the FWS only sparingly allowing public and private exceptions to the take prohibition.
Where take allowances are issued, numeric take limits poorly reflect the actual level of impact on a rusty patched bumble bee colony. The loss of an individual bee has an impact to the rest of the colony. Allowing the loss of a number or percentage of individuals has hidden conservation costs which fundamentally change what level is allowable under the jeopardy and likelihood of survival standards. Additionally, many impacts that would harm one bee or a small percentage of bees inherently harm the rest of the colony. Killing or harming one mammal in a population does not directly harm the rest of the population, which is why take allowances are usually numeric limits. However, the primary impacts to the rusty patched bumble bee directly caused by humans cannot be isolated to one bee. Parasite and pathogen exposure from commercial bumble bees affect an entire colony. Similarly, one worker bee can expose an entire colony to harmful or fatal pesticides by returning to the colony after contamination.[236] Take allowances should take these hidden impacts into consideration and recognize that a set numeric limit does not directly translate into an equal loss for the rusty patched bumble bee.
Take allowances should recognize and account for the differences between the different types and roles of rusty patched bumble bee. One method is to set different take allowances for the different types of bees based on their roles. Queen bees are always critical to the species. From the time that they breed to the time that they establish a colony, a queen bee represents an entire colony.[237] Take allowances should be incredibly strict for queen bees since the loss of one foundress is the loss of a whole colony for the season. They have slightly reduced significance in the fall, however, when new queens have yet to breed and go into hibernation. In a healthy population, the new queen would have close to 200 queen bees that hold the same genetic code and breeding potential.[238] While a new queen is raw potential rather than a seeded colony, male bees serve the same reproductive role and hold the same importance.[239] It would be appropriate to reflect the relative importance of male and new queen bees in comparison to foundress queens by allowing for slightly more take. Worker bees in a healthy colony do not hold individual significance.[240] However, sizable percentages of lost worker bees would have large impacts on colony growth and productivity. Take allowances could reflect the variable importance by setting a percentage of allowable worker bee deaths or creating a shifting numeric value based on colony size. There are other solutions that could account for the differences in roles within the rusty patched bumble bee. The important thing is that the differences in roles within the rusty patched bumble bee are considered and accounted for while setting take limits.
Providing numeric limits for whole colonies rather than individual bees is an alternative option to address the different roles and importance within a rusty patched bumble bee colony. The differences in importance between individual bees is a direct reflection of their contributions to the colony’s growth and productivity. A numeric limitation on the number of colonies could encapsulate different take limits for different types of bees. If this method was effectively embraced, a limitation of taking one colony would imply allowably taking one queen bee and only the number of new queens, males, and workers that would not endanger more than one colony. Although Sierra Club v. U.S. Department of Interior rejected a set take limit defined by a number of colonies, that determination was partially based on the lack of species data for the rusty patched bumble bee area and the non-quantitative limit of a “small percentage” of new queens.[241] The decision was not an inherent rejection of the representative use of colonies when setting take limits as a general principle.
The FWS is actively learning how to regulate the rusty patched bumble bee under the ESA. The take prohibition is a powerful legal protection and is therefore important to both the biological conservation and the legal protection of the rusty patched bumble bee.[242] There are opportunities to design incidental take allowances to be tailored to the rusty patched bumble bee’s needs. Understanding these areas is important for the purposes of setting, evaluating, and challenging upcoming take limits for the rusty patched bumble bee.
The decline in certain bumble bee species is an important environmental, economic, and legal issue that requires policy action. The ESA is the first and only federal law to provide specific legal protections to a eusocial bee. Carefully watching, analyzing, and influencing the rusty patched bumble bee’s management under the ESA creates opportunities for advocates to establish the ESA as a strong protection for other at-risk bumble bee species. Despite the historic bias against insects in implementing the ESA, proponents can intentionally use the ESA to provide specific protections that meet the unique management needs of the rusty patched bumble bee and any other colony bee species. Critical habitat and incidental take allowances are two protections under the ESA that could be leveraged to maximize protection and recovery for the rusty patched bumble bee.
Determining critical habitat for the rusty patched bumble bee in the immediate future is crucial to the species’ survival and recovery. When setting the geographic area for critical habitat the FWS needs to consider the intricacies of the species’ foraging needs, the effects of fragmented habitat on the species’ genetic health, the limited availability of the species’ nesting and hibernation sites, the diversity of ecoregions available as suitable habitat, and the need to embrace uncertainty for each of these considerations. To fully account for the rusty patched bumble bee’s habitat needs, the FWS likely needs to make a critical habitat of unprecedented size for insects. More important than the size, the agency must explicitly consider and account for each of the aforementioned habitat-based needs.
The FWS must also consider the rusty patched bumble bee’s unique biological features when setting take allowances for the species. Most importantly, the agency should be sensitive to the species’ vulnerability to genetic issues associated with small population sizes and strive to prevent existing populations from shrinking further. The FWS should also consider the inability of traditional numeric take limits to reflect the true costs of individual bees on colony health and the variability in importance of different bees with different roles.
Policymakers, litigators, judges, and parties interested in pollinator protection need to understand the specific biological characteristics that make managing the rusty patched bumble bee a new and interesting problem in the realm of environmental law. Understanding those qualities and intentionally integrating them into the rusty patched bumble bee’s management under the ESA would be a major step toward protecting the bees, supporting agricultural productivity, and preserving ecosystem health.
The existing size of proposed or final critical habitat for listed insect species.[243]
| Common Name | Scientific Name | Listing Status | Acres of Critical Habitat |
| Casey’s June Beetle | Dinacoma caseyi | Endangered | 678.9 |
| Comal Springs Dryopid Beetle | Stygoparnus comalensis | Endangered | 75.6 |
| Comal Springs Riffle Beetle | Heterelmis comalensis | Endangered | 28.2 |
| Delta Green Ground Beetle | Elaphrus viridis | Threatened | 969.4 |
| Helotes Mold Beetle | Batrisodes venyivi | Endangered | 546.7 |
| Beetle [no common name] | Rhandine exilis | Endangered | 1,938.5 |
| Beetle [no common name] | Rhadine infernalis | Endangered | 2,331.7 |
| Valley Elderberry Longhorn Beetle | Desmocerus californicus dimorphus | Threatened | 478.8 |
| Bartram’s Hairstreak Butterfly | Strymon acis bartrami | Endangered | 11,539 |
| Bay Checkerspot Butterfly | Euphydryas editha bayensis | Threatened | 17,759.1 |
| Fender’s Blue Butterfly | Icaricia icarioides fenderi | Endangered | 2,885.0 |
| Florida Leafwing Butterfly | Anaea troglodyte floridalis | Endangered | 10,561 |
| Island Marble Butterfly | Euchloe ausonides insulanus | Proposed | 813.0 |
| Mount Charleston Blue Butterfly | Icaricia (Plebejus) Shasta charlestonensis | Endangered | 5,270.3 |
| Oregon Silverspot Butterfly | Speyeria zerene hippolyta | Threatened | 361.4 |
| Palos Verdes Blue Butterfly | Glaucopsyche lydamus palosyerdesensis | Endangered | 74.7 |
| Quino Checkerspot Butterfly | Euphydryas editha quino | Endangered | 61,973.8 |
| Taylor’s Checkerspot | Euphydryas editha taylori | Endangered | 1,633.7 |
| Backline Hawaiian Damselfly | Megalagrion nigrohamatum nigrolineatum | Endangered | 3,1073.3 |
| Crimson Hawaiian Damselfly | Megalagrion leptodemas | Endangered | 3,1073.3 |
| Oceanic Hawaiian Damselfly | Megalagrion oceanicum | Endangered | 3,887.7 |
| Hine’s Emerald Dragonfly | Somatochlora hineana | Endangered | 25,890.5 |
| Hawaiian Picture-Wing Fly | Drosophila aglaia | Endangered | 295.2 |
| Hawaiian Picture-Wing Fly | Drosophila differens | Endangered | 988.2 |
| Hawaiian Picture-Wing Fly | Drosophila hemipeza | Endangered | 933.3 |
| Hawaiian Picture-Wing Fly | Drosophila heteroneura | Endangered | 4,571.8 |
| Hawaiian Picture-Wing Fly | Drosophila mulli | Threatened | 691.4 |
| Hawaiian Picture-Wing Fly | Drosophila musaphilia | Endangered | 793.6 |
| Hawaiian Picture-Wing Fly | Drosophila obatai | Endangered | 109.5 |
| Hawaiian Picture-Wing Fly | Drosophila ochrobasis | Endangered | 438.0 |
| Hawaiian Picture-Wing Fly | Drosophila substenoptera | Endangered | 324.0 |
| Hawaiian Picture-Wing Fly | Drosophila tarphytrichia | Endangered | 821.8 |
| Hawaiian Picture-Wing Fly | Drosophila sharpi | Endangered | 17,261.3 |
| Zayante Band-Winged Grasshopper | Trimerotropis infantilis | Endangered | 11,151.5 |
| Blackburn’s Sphinx Moth | Manduca blackburni | Endangered | 3,172.1 |
| Ash Meadows Naucorid | Ambrysus amargosus | Threatened | 0.3 |
| Hawaiian Picture-Wing Fly | Drosophila montgomeryi | Endangered | 821.8 |
| Hawaiian Picture-Wing Fly | Drosophila neoclavisetae | Endangered | 137.2 |
| Dakota Skipper | Hesperia dacotae | Threatened | 19,976.5 |
| Laguna Mountains Skipper | Pyrgus ruralis lagunae | Endangered | 6,250.7 |
| Powershiek Skipperling | Oarisma powershiek | Endangered | 25,933.8 |
| Salt Creek Tiger Beetle | Cicindela nevadica lincolniana | Endangered | 852.3 |
- * J.D. Candidate, 2020, University of Colorado Law School. The author would like to thank the wonderful staff of the Colorado Natural Resources, Energy & Environmental Law Review for their work and Professors Sean Helle and Amy Griffin for their support and guidance on this Note. The author would like to specially thank Professor Ian Kaplan for sparking his interest in the fascinating world of insect ecology. ↑
- Endangered and Threatened Wildlife and Plants; Endangered Species Status for Rusty Patched Bumble Bee, 82 Fed. Reg. 27, 10285 (Feb. 10, 2017) (to be codified at 50 C.F.R. pt. 17) [hereinafter Rusty Patched Listing Delay]. ↑
- Dan Mullen, Rusty-Patched Bumble Bee, Flicker (Jul. 30, 2015),
https://www.flickr.com/photos/8583446@N05/28469388786/in/photostream/. ↑
- See discussion infra Section Bumble Bee Decline. ↑
- Jeff Ollerton, Rachael Winfree & Sam Tarrant, How Many Flowering Plants are Pollinated by Animals?, 120 Oikos 321, 322–23 (2011). ↑
- Christopher M. Lambe, What’s All the Buzz About? Analyzing the Decision to List the Rusty Patched Bumblebee on the Endangered Species List, 29 Vill. Envtl. L. J. 129, 135 (2018); Pollinators Need You. You Need Pollinators, Pollinator P’ship, http://www.pollinator.org/pollinators.htm (last visited Feb. 4, 2017). ↑
- Sarina Jepsen et al., The Xerces Soc’y for Invertebrate Conservation, Petition to List the Rusty Patch Bumblebee Bombus Affinis (Cresson), 1863 As an Endangered Species Under the U.S. Endangered Species Act 27 (2013). ↑
- Id. ↑
- Stephen L. Buchmann, Bees Use Vibration to Aid Pollen Collection from Non-poricidal Flowers, 58 J. Kan. Entomological Soc’y 517, 517 (1985). ↑
- Sarah A. Corbet et al., Temperature and the Pollinating Activity of Social Bees, 18 Ecological Entomology 17, 28 (1993). ↑
- Alexanda-Maria Klein et al., Importance of Pollinators in Changing Landscapes for World Crops, 274 Proc. Royal Soc’y B 303, 306 (2006). ↑
- Lambe, supra note 5, at 135; See also Pollinators, U.S. Fish & Wildlife Serv., https://www.fws.gov/pollinators/ (last updated June 18, 2019). ↑
- David Inouye, Samuel Droege & Jonathan Mawdsley, Words Alone Will Not Protect Pollinators, 355 Science 357, 357 (2017). ↑
- Oils found in plants promoted by animal pollination contain seventy-four percent of lipids required for human health and are the primary source of fat-soluble vitamins. Notably, ninety-eight percent of available vitamin C comes from citrus and other fruits and vegetables which animals pollinate. Elisabeth J. Eilers et al., Contribution of Pollinator-Mediated Crops to Nutrients in the Human Food Supply, PLoS One, June 22, 2011 at 1, 2. ↑
- Kent P. McFarland & Leif Richardson, Rusty-Patched Bumble Bee (Bombus affinis): Report to the Vermont Endangered Species Committee 4 (2013); Jepsen et al., supra note 6, at 28. ↑
- In Britain, a fifty-two percent reduction in bee richness correlates with a twenty-two percent reduction in the distribution of plant species pollinated by bees. A similar correlation exists in the Netherlands where bee richness fell by sixty-seven percent and the distribution of plant species pollinated by bees fell by twelve percent. J. C. Biesmeijer et al., Parallel Declined in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands, 313 Science 351 (2006). ↑
- Jane Memmott, Nickolas M. Waser & Mary V. Price, Tolerance of Pollination Networks to Species Extinctions, 271 Proc. Royal Soc’y Series B 2605, 2606 (2004). ↑
- See infra notes 17–18 and accompanying text. ↑
- Dale F. Schweitzer, Nicole A. Capuano, Bruce E. Young & Sheila R. Colla, U.S. Dep’t of Agric. Forest Serv. & NatureServe, Conservation and Management of North American Bumble Bees 3 (2012). ↑
- Id. at 16–17. ↑
- Jonathan B. Koch, The Decline and Conservation Status of North American Bumble Bees 3 (Aug. 2011) (unpublished M.S. thesis, Utah State University) (on file with the Digital Commons, Utah State University). ↑
- Id. at 3. ↑
- Jepsen et al., supra note 6, at 26–27. ↑
- See infra Section The Rusty Patched Bumble Bee’s Life Cycle. ↑
- Jepsen et al., supra note 6, at 26–27. ↑
- Id. at 10. ↑
- Richard G. Hatfield & Gretchen LeBuhn, Patch and Landscape Factors Shape Community Assemblage of Bumble Bees, Bombus spp. (Hymenoptera: Apidae), in Montane Meadows, 139 Biological Conservation 150, 155 (2007). ↑
- Madhumita Bhattacharya et al., Are Roads and Railroads Barriers to Bumblebee Movement in a Temperate Suburban Conservation Area?, 109 Biological Conservation 37, 44 (2003). ↑
- Jonathan B. Koch, et al., US Bombus, a Database of Contemporary Survey Data for North American Bumble Bees (Hymenoptera, Apidae, Bombus) Distributed in the United States, Biodiversity Data J., 30 Dec 2015, at 1, 6. ↑
- Jepsen et al., supra note 6, at 23. ↑
- James D. Thomson, Flowering Phenology, Fruiting Success and Progressive Deterioration of Pollination in an Early-Flowering Geophyte, 365 Phil. Transactions of the Royal Soc’y B 3187, 3197 (2010). ↑
- S. Cameron et al., North American Bumble Bee Species Conservation Planning Workshop Final Report 21, 37 (2010). ↑
- McFarland & Richardson, supra note 14, at 8. ↑
- D. Goulson, G. C. Lye & B. Darvill, Decline and Conservation of Bumble Bees, 53 Ann. Rev. of Entomology 191, 194 (2008). ↑
- Sheila R. Colla & Laurence Packer, Evidence for Decline in Eastern North American Bumblebees (Hymenoptera: Apidae), with Special Focus on Bombus Affinis Cresson, 17 Biodiversity and Conservation 1379, 1388 (2008). ↑
- Penelope R. Whitehorn et al., Neonicotinoid Pesticide Reduces Bumble Bee Colony Growth and Queen Production, 336 Science 351, 351 (2012). ↑
- McFarland & Richardson, supra note 14, at 6. ↑
- See Hayo H.W. Velthuis & Adriaan van Doorn, A Century of Advances in Bumblebee Domestication and the Economic and Environmental Aspects of its Commercialization for Pollination, 37 Apidologie 421, 426, 429, 433 (2006). ↑
- See McFarland & Richardson, supra note 14, at 6. ↑
- Rosemary L. Malfi & T’ai H. Roulston, Patterns of Parasite Infection in Bumble Bees (Bombus spp.) of Northern Virginia, 39 Ecological Entomology 17, 24 (2014). ↑
- Jepsen et al., supra note 6, at 22. ↑
- Endangered and Threatened Wildlife and Plants; Endangered Species Status for Rusty Patched Bumble Bee, 82 Fed. Reg. 3186, 3190 (Jan. 11, 2017) (to be codified at 50 C.F.R. pt. 17) [hereinafter Rusty Patched Listing]. ↑
- See Emily Knobbe, Honeybees and the Law: Protecting Our Pollinators, 30 J. Envtl. L. & Litig. 219, 234–36 (2015). ↑
- Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) and Federal Facilities, Envtl. Prot. Agency https://www.epa.gov/enforcement/federal-insecticide-fungicide-and-rodenticide-act-fifra-and-federal-facilities (last updated Jan. 29. 2019). ↑
- Schedule for Review of Neonicotinoid Pesticides, Envtl. Prot. Agency, https://www.epa.gov/pollinator-protection/schedule-review-neonicotinoid-pesticides (last updated on Jul. 22, 2019). ↑
- Jepsen et al., supra note 6, at 20. ↑
- See id. at 18. ↑
- See id. ↑
- See id. ↑
- See id. ↑
- U.S. Fish and Wildlife Serv., ESA Basics (2013), https://www.fws.gov/endangered/esa-library/pdf/ESA_basics.pdf [hereinafter ESA Basics]. ↑
- Id. ↑
- 16 U.S.C. § 1532(6) (2018). ↑
- 16 U.S.C. § 1533(a)(1) (2018). ↑
- ESA Basics, supra note 50. ↑
- 16 U.S.C. § 1533(a)(3) (2018). ↑
- Thomas F. Darin, Designating Critical Habitat Under the Endangered Species Act: Habitat Protection Versus Agency Discretion, 24 Harv. Envtl. L. Rev. 209, 218–19 (2000). ↑
- Hawaiian Yellow-Faced Bees, Xerces Soc’y, https://xerces.org/hawaiian-yellow-faced-bees/ (last visited Jan. 1, 2019). ↑
- Rusty Patched Listing, supra note 41, at 3186. ↑
- U.S. Fish and Wildlife Serv., National Listing Workplan, 7 Year-Workplan (September 2016 Version), (2016), https://www.fws.gov/endangered/esa-library/pdf/Listing%207-Year%20Workplan%20Sept%202016.pdf; U.S. Fish and Wildlife Serv., National Listing Workplan, Unscheduled Listing Actions (September 2016 Version) (2016), https://www.fws.gov/endangered/esa-library/pdf/Unscheduled%20Listing%20Actions%20Sept%202016.pdf. ↑
- Endangered and Threatened Wildlife and Plants; Endangered Species Status for Franklin’s Bumble Bee, 84 Fed. Reg. 40006 (Aug. 13, 2019) (to be codified at 50 C.F.R. pt. 17) [hereinafter Franklin Proposed Listing]. The FWS based the proposed listing primarily on suspected impacts from pathogens—factor three for listing—and the inadequacy of existing regulatory mechanisms to reduce the existing threats to the Franklin’s bumble bee—factor four for listing. Id. at 40015. ↑
- 16 U.S.C. § 1536(a)(2) (2018). ↑
- 16 U.S.C. § 1538(a) (2018). ↑
- 16 U.S.C. § 1532(19) (2018). ↑
- See Paul Boudreaux, Understanding Take in the Endangered Species Act, 34 Ariz. St. L. J. 733 (2002), for a discussion of some of the cases that explore the boundaries of “take.” ↑
- Conor P. McGowan & Mark R. Ryan, Arguments for Using Population Models in Incidental Take Assessments for Endangered Species, 1(2) J. of Fish and Wildlife Mgmt. 183, 184 (2010). ↑
- 16 U.S.C. § 1539 (2012). ↑
- Id. ↑
- 16 U.S.C. § 1540(g) (2012). ↑
- McFarland & Richardson, supra note 14, at 12. ↑
- See Scott Hoffman Black et al., Endangered Invertebrates: the Case for Greater Attention to Invertebrate Conservation, 18(2) Endangered Species UPDATE 42, 45 (2001). ↑
- Boudreaux, supra note 64, at 773. ↑
- Black et al., supra note 70, at 44. ↑
- Scott Hoffman Black & D. Mace Vaughan, Endangered Insects, in Encyclopedia of Insects 320, 321 (Vincent H. Resh & Ring T. Cardé eds. 2nd ed. 2009). ↑
- David S. Wilcove & Lawerence L. Master, How many endangered species are there in the United States?, 3(8) Frontiers in Ecology and the Env’t 414, 415 (2005). ↑
- Black et al., supra note 70, at 44. ↑
- Koch, supra note 20, at 82. ↑
- Jonathan B. Koch & James P. Strange, Constructing a Species Database and Historic Range Maps for North American Bumblebees (Bombus Sensu Stricto Latreille) to Inform Conservation Decisions, 9(3) Uludag Bee J. 97, 99 (2009). ↑
- See Jepsen et al., supra note 6, at 4. ↑
- Rusty Patched Listing, supra note 41, at 3187. ↑
- Regional tallgrass prairie losses include: 99.999% loss in the Grand Prairie area of Mississippi Alluvial Plains in Arkansas, 99% loss east of the Missouri River, 85% loss west of the Missouri River, greater than 99.9% loss in Iowa, 99% to greater than 99.9% loss in Illinois, greater than 99% loss in Indiana, 100% loss of black silt-loam and gravel hill tallgrass prairies of Indiana, 99.5% loss in Missouri, greater than 97% loss in the eastern one-third of Nebraska, 82% loss in Kansas, and greater than 99% loss in Montana. Reed F. Noss, Edward T. LaRoe III, J. Michael Scott & Nat’l Biological Serv., Endangered Ecosystems of the United States: A Preliminary Assessment of Loss and Degradation 39, 43 (Paul A. Opler, et. al. eds., 1995). ↑
- Rusty Patched Listing, supra note 41, at 3189. ↑
- Jepsen et al., supra note 6, at 4. ↑
- Rusty Patched Listing, supra note 41, at 3188. ↑
- Id. ↑
- Id. ↑
- Id. at 3186. ↑
- Rusty Patched Listing, supra note 41, at 10285. ↑
- Rusty Patched Listing, supra note 41, at 3186. ↑
- Id. at 3207. ↑
- Id. ↑
- Id. ↑
- See id. ↑
- See infra note 161. ↑
- Rusty Patched Listing, supra note 41, at 3208. ↑
- Recovery of a species is an explicit goal of the ESA. See supra notes 48 and 50 and accompanying text. ↑
- Rusty Patched Listing, supra note 41, at 3187. ↑
- Id. ↑
- Goulson & Darvill, supra note 33, at 194. ↑
- Rusty Patched Listing, supra note 41, at 3187. ↑
- Koch & Strange, supra note 77, at 99 (identifying a mean of 1,081 workers and males per colony in a single season). ↑
- Id. (identifying a mean production of 181 new queens per colony in a single season). ↑
- Rusty Patched Listing, supra note 41, at 3187. ↑
- Id. ↑
- Id. ↑
- Jepsen et al., supra note 6, at 25–26. ↑
- Id. at 25. ↑
- Id. ↑
- Koch & Strange, supra note 77, at 99. ↑
- Jepsen et al., supra note 6, at 12. ↑
- Paul Williams et al., Bumblebee Vulnerability: Common Correlates of Winners and Losers Across Three Continents, 23 Conservation Biology 931, 935 (2009). ↑
- Jepsen et al., supra note 6, at 10. ↑
- Rusty Patched Listing, supra note 41, at 3204–05. ↑
- Heather M. Hines & Stephen D. Hendrix, Bumble Bee (Hymenoptera: Apidae) Diversity and Abundance in Tallgrass Prairie Patches: Effects of Local and Landscape Floral Resources, 34 Envtl. Entomology 1477, 1481 (2005). ↑
- Env’t. and Climate Change Can., Recovery Strategy for the Rusty-Patched Bumble Bee (Bombus Affinis) In Canada [Proposed] 28 (2016). ↑
- Id. at 8. ↑
- Koch & Strange, supra note 77, at 99. ↑
- McFarland & Richardson, supra note 14, at 4. ↑
- Env’t. and Climate Change Can., supra note 114, at 6. ↑
- Id. at 7. ↑
- Jepsen et al., supra note 6, at 23. ↑
- Rusty Patched Listing, supra note 41, at 3187. ↑
- McFarland & Richardson, supra note 14, at 3–4. ↑
- Rusty Patched Listing, supra note 41, at 3191. ↑
- Olivier Lepais et al., Estimation of Bumble Bee Queen Dispersal Distances Using Sibship Reconstruction Method, 19 Molecular Ecology 819, 827 (2010). ↑
- McFarland & Richardson, supra note 14, at 10. ↑
- Env’t. and Climate Change Can., supra note 114, at 16–17. ↑
- Id. at 34. ↑
- Jepsen et al., supra note 6, at 22. ↑
- Rusty Patched Listing, supra note 41, at 3190. ↑
- Env’t. and Climate Change Can., supra note 114, at 5. ↑
- B. Darvill et al., Triploid Bumblebees Indicate a Direct Cost of Inbreeding in Fragmented Populations, 21 Molecular Ecology 3988, 3993 (2012). ↑
- Jepsen et al., supra note 6, at 22. ↑
- Amro Zayed & Laurence Packer, Complementary Sex Determination Substantially Increases Extinction Proneness of Haplodiploid Populations, 102(30) PNAS 10742, 10744, 10745 fig. 3 (2005). ↑
- Id. at 10742. ↑
- Darvill et al., supra note 131, at 3988–89. ↑
- Penelope R. Whitehorn et al., Kin Recognition and Inbreeding Reluctance in Bumblebees, 40 Apidologie 627, 628 (2009). ↑
- Zayed & Packer, supra note 133, at 10745–46. ↑
- 16 U.S.C. § 1532(5)(A) (2018). ↑
- 50 C.F.R. § 424.02 (2019). ↑
- 16 U.S.C. § 1532(5)(A) (2018). ↑
- Kalyani Robbins, Recovery of an Endangered Provision: Untangling and Revising Critical Habitat Under the Endangered Species Act, 58 Buff. L. Rev. 1095, 1105 (2010). ↑
- Id. at 1097. ↑
- 16 U.S.C. § 1533(a)(3) (2018). ↑
- 16 U.S.C. § 1533(b)(2) (2018). ↑
- See Darin, supra note 56, at 218; 16 U.S.C. § 1533(b)(2). ↑
- See Darin, supra note 56, at 218; 16 U.S.C. § 1533(b)(2). ↑
- Jason M. Patlis, Paying Tribute to Joseph Heller with the Endangered Species Act: When Critical Habitat Isn’t, 20 Stan. Envtl. L. J. 133, 136 (2001). ↑
- Id. at 142. ↑
- Robbins, supra note 141, at 1096. ↑
- Nat’l Research Council, Science and the Endangered Species Act 73 (1995). ↑
- Patlis, supra note 147, at 141. ↑
- Nat’l Research Council, supra note 150, at 72. ↑
- Rusty Patched Listing, supra note 41, at 3207. ↑
- Id. at 3206. ↑
- ESA Basics, supra note 50. ↑
- 16 U.S.C. § 1536(a)(2) (2018); ESA Basics, supra note 50. ↑
- 16 U.S.C. § 156(b)(3)(A) (2018); Rusty Patched Listing, supra note 41, at 3206. ↑
- Robbins, supra note 141, at 1105. ↑
- Erik Harvey et al., Recovery Plan Revisions: Progress or Due Process?, 12 Ecological Applications 682, 688 (2002). ↑
- Carolyn J. Lundquist et al., Factors Affecting Implementation of Recovery Plans, 12 Ecological Applications 713, 715 (2002). ↑
- Oliver A. Houck, The Endangered Species Act and Its Implementation by the U.S. Departments of Interior and Commerce, 64 U. Colo. L. Rev. 277, 311 (1993). ↑
- Id. ↑
- Id. ↑
- Id. ↑
- See id. at 308. ↑
- Martin F. Taylor et al., The Effectiveness of the Endangered Species Act: A Quantitative Analysis, 55 BioScience 360, 362 (2005). ↑
- Nat’l Research Council, supra note 150, at 72. Only 596 species of the 1959 species listed as either endangered or threatened had critical habitat designations as of 2010. See Robbins, supra note 141, at 1107. ↑
- See 50 C.F.R. § 17.95(i) (2018). ↑
- Franklin Proposed Listing, supra note 60, at 40018. ↑
- Robbins, supra note 141, at 1106. ↑
- Id. ↑
- Darin, supra note 56, at 221. ↑
- Id. at 228. ↑
- See id. ↑
- Rusty Patched Listing, supra note 41, at 3207. ↑
- Id. ↑
- See National Listing Workplan, U.S. Fish and Wildlife Serv. (last updated July 1, 2019), https://www.fws.gov/endangered/esa-library/pdf/5-Year%20Listing%20Workplan%20May%20Version.pdf. ↑
- Lucas Rhoads, Protecting the Rusty Patched Bumble Bee: Round Three, NRDC (Jan. 17, 2019), https://www.nrdc.org/experts/lucas-rhoads/third-times-charm-nrdc-sues-again-protect-rpbb; NRDC Complaint Challenging Failure to Designate Critical Habitat for the Rusty Patched Bumble Bee, NRDC (Jan. 17, 2019), https://www.nrdc.org/resources/nrdc-complaint-challenging-failure-designate-critical-habitat-rusty-patched-bumble-bee. ↑
- See supra Section Habitat-Related Needs. ↑
- See supra text accompanying notes 70–75. ↑
- Although butterflies are not eusocial species, they are pollinators and can function as a general point of comparison to the rusty patched bumble bee. Bay checkerspot butterfly’s critical habitat is divided into thirteen units across two California counties. Bartram’s hairstreak butterfly critical habitat is divided into seven units across two Florida counties. The units range from 97 to 7,994 acres in size. Fender’s blue butterfly’s critical habitat is divided into thirteen units across four Oregon counties. Florida leafwing butterfly critical habitat is divided into four units across two Florida counties. The units range from 296 to 7,994 acres. Mount Charleston blue butterfly critical habitat consists of three small patches in one concentrated area of Nevada. Oregon Silverspot butterfly critical habitat is one patch of land in Oregon. Palos Verdes blue butterfly critical habitat consists of three small zones in one concentrated area of California. The Quino checkerspot butterfly critical habitat is divided into ten units across two California counties. 50 C.F.R. § 17.95(i) (2018); See 50 C.F.R. § 17.95(i) (2018) for more detailed information and maps of the critical habitat designations; See infra Table 1. for the total size of each critical habitat designation. ↑
- See U.S. FWS Threatened & Endangered Species Active Critical Habitat Report, Environmental Conservation Online System, U.S. Fish and Wildlife Serv. (last visited on Feb. 2, 2019), https://ecos.fws.gov/ecp/report/table/critical-habitat.html. ↑
- Id. ↑
- See supra note 181. ↑
- See supra notes 77 and 79 and accompanying text. ↑
- 16 U.S.C. § 1532(5)(A) (2012). ↑
- ESA Basics, supra note 50. ↑
- See supra notes 113-18 and accompanying text. ↑
- See supra note 101 and accompanying text. ↑
- See supra notes 112–13 and accompanying text. ↑
- See supra note 27 and accompanying text. ↑
- See supra Section Genetic Needs. ↑
- See supra notes 130–32 and accompanying text. ↑
- See supra note 131 and accompanying text. ↑
- See supra notes 121–27 and accompanying text. ↑
- Id. ↑
- See supra note 105 and accompanying text. ↑
- See supra note 81 and accompanying text. ↑
- Rusty Patched Listing, supra note 41, at 3188. ↑
- See supra note 124 and accompanying text. ↑
- See supra note 55 and accompanying text. ↑
- Patlis, supra note 147, at 141. ↑
- 16 U.S.C. § 1538(a) (2018). ↑
- Boudreaux, supra note 64, at 733. ↑
- 16 U.S.C. § 1532(19) (2018). ↑
- 50 C.F.R. § 17.3 (2019). ↑
- Id. ↑
- Boudreaux, supra note 64, at 733. ↑
- J. B. Ruhl, How to Kill Endangered Species, Legally: The Nuts and Bolts of Endangered Species Act HCP Permits for Real Estate Development, 5 Envtl. L. 345, 360–61 (1999). ↑
- 16 U.S.C. § 1540(a) (2018) (civil penalties); 16 U.S.C. § 1540(b) (2018) (criminal penalties). ↑
- 16 U.S.C. § 1540(e)(6) (2018). ↑
- McGowan & Ryan, supra note 65, at 183. ↑
- 16 U.S.C. § 1539(a)(1)(B) (2018). ↑
- McGowan & Ryan, supra note 65, at 184. ↑
- 16 U.S.C. § 1539(a)(2)(B)(iv) (2018). ↑
- McGowan & Ryan, supra note 65, at 184. ↑
- Ruhl, supra note 209, at 351. ↑
- Houck, supra note 161, at 317. ↑
- Id. at 318. ↑
- Id. ↑
- McGowan & Ryan, supra note 65, at 183. ↑
- Boudreaux, supra note 64, at 736. ↑
- Ruhl, supra note 209, at 382; 16 U.S.C. § 1539(a)(2)(B) (2018). ↑
- Ruhl, supra note 209, at 382; 16 U.S.C. § 1539(a)(2)(B) (2018). ↑
- Ruhl, supra note 209, at 391. ↑
- See, e.g., Petzel v. Kane Cty. Dep’t of Transp., 2018 WL 3740629 (N.D. Ill. 2018) (dismissing the claim for failing to meet the notice requirement); Strahan v. Nielsen, 2018 WL 3966318 (D.N.H. 2018) (dismissing the claim for failing to meet the notice requirement). ↑
- Sierra Club v. U.S. Dep’t of the Interior, 899 F.3d 260, 267 (4th Cir. 2018). ↑
- Id. at 267. ↑
- Id. at 276. ↑
- Id. at 276–77. ↑
- See supra notes 128–31 and accompanying text. ↑
- See supra notes 105–07 and accompanying text. ↑
- See supra notes 128–31 and accompanying text. ↑
- See supra note 131 and accompanying text. ↑
- Bumble bees expose themselves to pesticides by consuming contaminated nectar or gathering contaminated pollen. Contaminated pollen can be brought back to the colony and cause negative sub-lethal effects such as reduced male production, reduced egg hatch, reduced queen production, and other reductions in different elements of colony productivity and health. Jennifer Szymanski et al., U.S. Fish and Wildlife Serv., Rusty Patched Bumble Bee (Bombus affinis) Species Status Assessment 43 (2016). ↑
- See supra Section The Rusty Patched Bumble Bee’s Life Cycle. ↑
- See supra note 101 and accompanying text. ↑
- See id. ↑
- See supra notes 100 and 106 and accompanying text. ↑
- Sierra Club v. U.S Dep’t of Interior, 899 F.3d at 277. ↑
- See supra note 204 and accompanying text. ↑
- U.S. FWS Threatened & Endangered Species Active Critical Habitat Report, Environmental Conservation Online System, U.S. Fish and Wildlife Serv. (last visited on Feb. 2, 2019), https://ecos.fws.gov/ecp/report/table/critical-habitat.html.; 50 C.F.R. § 17.95(i) (2018) (Data for the Bartram’s hairstreak butterfly and Florida leafwing butterfly). ↑